- Navigating Your Midlife Crisis: Embracing New Possibilities
- City Raccoons Showing Signs of Domestication
- Mapping the Exposome: Science Broadens Focus to Environmental Disease Triggers
- One Week Less on Social Media Linked to Better Mental Health
- Your Brain Changes in Stages as You Age, Study Finds
- Some Suicide Victims Show No Typical Warning Signs, Study Finds
- ByHeart Formula Faces Lawsuits After Babies Sickened With Botulism
- Switch to Vegan Diet Could Cut Your Greenhouse Gas Emissions in Half
- Regular Bedtime Does Wonders for Blood Pressure
- Dining Alone Could Mean Worse Nutrition for Seniors
FDA Warns Against ‘Off-Label’ Use of Pfizer Vaccine in Younger Children
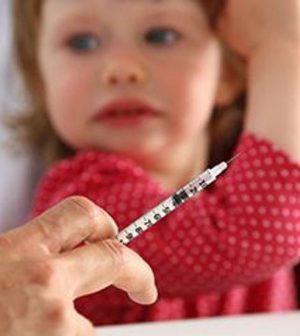
“Off-label” use of the Pfizer coronavirus vaccine in children younger than 12 is “not appropriate,” the U.S. Food and Drug Administration warned Monday. Off-label use refers to an approved medicine being used in ways or in patients it’s not FDA-approved for.
The American Academy of Pediatrics (AAP) also “strongly discourages” such use.
On Monday, the vaccine was fully approved for use in people 16 and older. It also has emergency use authorization for use in people as young as 12, but it is still is not cleared for use in younger children.
“We do not have data on the proper dose nor do we have full data on the safety in children younger than what is in the EUA [emergency use authorization],” Acting FDA Commissioner Dr. Janet Woodcock said Monday.
“So, that would be a great concern that people would vaccinate children because we don’t have the proper dose and we don’t have the safety data, nor do we have all the efficacy data, as well,” Woodcock said, CNN reported. “We are not recommending that children younger than age 12 be vaccinated with this vaccine. It would not be appropriate.”
While many parents want to get their younger children vaccinated, Woodcock pointed out that children “are not just small adults.”
“We really would have to have the data and the appropriate dose before recommending that children be vaccinated,” she explained.
“The clinical trials for the COVID-19 vaccine in children ages 11 years old and younger are underway, and we need to see the data from those studies before we give this vaccine to younger children,” AAP President Dr. Lee Savio Beers said in a statement Monday.
The adult vaccine dose is much higher than doses being tested in younger children and the AAP “strongly discourages” off-label use of the vaccine in children younger than 12, the statement read.
Pfizer has said it expects to have vaccine trial data on children ages 5 to 11 by the end of September, and data for children ages 2 to 5 could be available shortly after, CNN reported.
Moderna and Johnson & Johnson are also conducting trials of their vaccines in children, CNN reported.
As of last week, the AAP had reported 180,000 new cases of COVID-19 among children and adolescents. So far, about 8.5 million, or 34% of all adolescents, are fully vaccinated.
More information
Visit the U.S. Food and Drug Administration for more on the Pfizer vaccine approval.
SOURCE: CNN News
Source: HealthDay
Copyright © 2025 HealthDay. All rights reserved.










