- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
B 8/10 — FDA Warns Amazon, Other Vendors About Sale of Skin Tag Removal Products
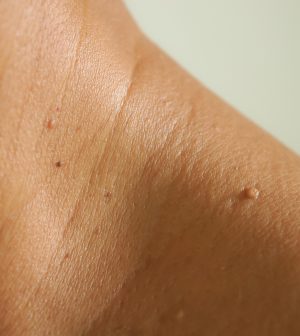
WEDNESDAY, Aug. 10, 2022 (HealthDay News) – The U.S. Food and Drug Administration on Tuesday issued warning letters to three companies, including Amazon, for selling unapproved products for removing moles and skin tags.
No over-the-counter medications have FDA approval for that purpose, and the Food, Drug, and Cosmetic Act prohibits interstate sale of unapproved drugs and cosmetics.
“It is the FDA’s duty to protect public health from harmful products not approved for the U.S. marketplace,” said Donald Ashley, director of the compliance office in the FDA’s Center for Drug Evaluation and Research. “This includes where online retailers like Amazon are involved in the interstate sale of unapproved drug products. We will continue to work diligently to ensure that online retailers do not sell products that violate federal law.”
Besides Amazon, the FDA issued warning letters to Ariella Naturals and Justified Laboratories.
The agency emphasized that it has not examined the mole and skin tag removal products sold by these companies for safety, effectiveness or quality.
The warning letters put the companies on notice that failure to address the violations cited by the FDA may result in legal action, including seizure. Once they receive the warning letter, the companies have 15 days to inform the agency of any corrective measures they have taken.
The agency warned earlier this summer that using unregulated products for do-it-yourself removal of moles, skin tags and another type of growth known as seborrheic keratoses can mask skin cancer and result in wounds and scarring.
“There are several reasons that patients should avoid trying to treat moles at home. And that is certainly the most concerning… that cancer patients often mistake skin cancer for benign moles,” Dr. Chad Prather, a board-certified dermatologist in Baton Rouge, La, said at the time. “We commonly see patients who have a skin cancer. It’s been diagnosed and their initial process was to try to treat that at home with either physical means or sometimes these over-the-counter products.”
That can cloud the diagnosis of very serious skin cancers, such as melanoma, Prather said.
“We judge how bad a melanoma is by how deep it goes. And we really need that initial biopsy to know the true depth so that we can choose the most appropriate treatment method, whether it’s surgery or checking lymph nodes or followed by immunotherapy,” Prather explained.
The FDA said Tuesday that it will continue to use all the tools available to protect public health and remove fraudulent or unproven drug products from the American market. The warning letters were just the first step.
Consumers and medical professionals are asked to report any adverse events related to the products to the MedWatch Adverse Event Reporting.
More information
BeSafeRx is a U.S. Food and Drug Administration site for online pharmacy information.
SOURCE: U.S. Food & Drug Administration, news release, Aug. 9, 2022
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










