- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
FDA Calls for Better Accuracy of Pulse Oximeters in People of Color
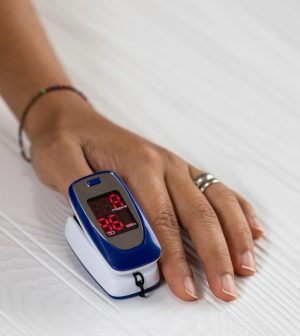
Pulse oximeters — those tiny devices that measure blood oxygen levels with a quick clip to your finger — may soon get a major upgrade to ensure they work just as well for people of all skin tones.
The U.S. Food and Drug Administration (FDA) released draft guidelines on Jan. 6 proposing that manufacturers conduct larger, more inclusive studies to ensure these devices, which became essential during COVID-19, work for people with darker skin.
Among the key changes, the new recommendations call for:
-
Enrolling at least 150 patients with diverse skin tones in clinical studies
-
Increasing representation of patients with darker skin to at least 25% in each study, up from 15%
-
Using multiple methods to evaluate skin pigmentation, including scientific measurements of melanin
The proposal applies only to professional pulse oximeters used in medical settings, such as hospitals and clinics. Over-the-counter oximeters, often marketed as “general wellness” devices, are not affected.
The FDA’s recommendations come after studies, including one from 2021, found that pulse oximeters often overestimate oxygen levels in Black patients, potentially delaying treatment and increasing health risks, a news release states.
Existing devices already on the market will not need to meet these new standards unless manufacturers request updates or modifications.
The FDA will accept public comments on the draft proposal for 60 days before finalizing the guidelines.
More information
The American Lung Association has more on pulse oximetry.
SOURCE: The U.S. Food and Drug Administration (FDA), federal proposal draft, Jan. 6; AP News
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










