- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
FDA Upgrades Recall on 160,000+ Bottles of Thyroid Medication
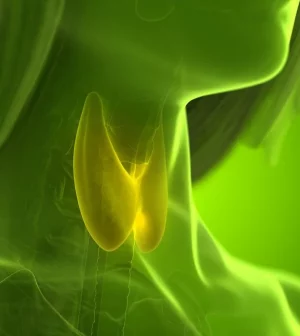
The U.S. Food and Drug Administration (FDA) has upgraded a recall of a commonly prescribed thyroid medication due to what it described as “subpotent” active ingredients.
The recall of more than 160,000 bottles of levothyroxine sodium, which went into effect June 20, was upgraded to a Class II recall on July 23. Class II recalls occur when use of the drug poses a moderate public health risk, according to the FDA.
The recall was initiated because the medication content of the recalled pills is “below the approved specification,” according to the New York-based television network NTD.
No other details were available about the recall, including whether anyone has become ill from the weak pills.
Levothyroxine sodium is used to treat a sluggish thyroid gland (hypothyroidism). Left untreated, hypothyroidism can lead to heart problems and high cholesterol, among other issues, according to the Mayo Clinic.
The recalled drugs are manufactured by India-based Intas Pharmaceuticals for North Carolina-based Accord Healthcare, NTD reported. They total 160,630 bottles and come in various doses, strengths, and packages, including:
-
Lot D2400536; expiration date, Feb. 28, 2026
-
Lot D2300325; expiration date, Jan. 31, 2026
-
Lot D2400679; expiration date, Feb. 28, 2026
-
Lot D2300087; expiration date, Dec. 31, 2025
-
Lot D2300092; expiration date, Dec. 31, 2025
-
Lot D2400722; expiration date, March 31, 2026
-
Lot D2300104; expiration date, Dec. 31, 2025
-
Lot D2300076; expiration date, Dec. 31, 2025
-
Lot D2300042; expiration date, Dec. 31, 2025
More information
More details on the recalled thyroid pills can be found on the FDA’s website.
SOURCES: U.S. Food and Drug Administration, alert, July 23, 2025; NTD, July 23, 2025
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










