- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
- Understanding the Connection Between Anxiety and Depression
- How Daily Prunes Can Influence Cholesterol and Inflammation
Scientists Grow ‘Model Brain’ From Stem Cells

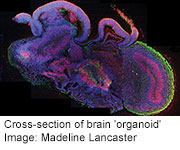
WEDNESDAY, Aug. 28In another milestone for regenerative medicine, Austrian scientists report they have turned stem cells into a collection of tissues that resembles the beginnings of the human brain.
The tiny organoids, as the researchers call them, grow to around 4 millimeters in size. They have many of the same specialized regions that are seen in fetal brains around nine weeks into development.
Though these baby brains aren’t likely to ever work as replacement parts, they are useful for understanding brain development, and where and how it can go awry.
Researchers have already used the model to better understand one problem called microcephaly, a genetic condition that causes a child to be born with smaller-than-average head and brain, and results in mental disability.
The new brain-growing method may also be useful for studying other neurological disorders, such as schizophrenia and autism, and for testing new drugs, the researchers said in a news conference Tuesday.
The study was published online Aug. 28 in the journal Nature.
Previously, researchers have coaxed stem cells to grow into the beginnings of a human eye and a functioning human liver. They’ve also created working pituitary glands and beating hearts for mice.
“But so far, the most complex of human organs, the human brain, has not been susceptible to these type of cultures,” said researcher Juergen Knoblich, deputy scientific director of the Institute of Molecular Biotechnology at the Austrian Academy of Science, in Vienna.
For the new study, researchers bathed stem cells in growth factors to encourage them to divide. When the stem cells had formed tiny balls of cells and the beginnings of nerve tissue, they were implanted in droplets of a gel protein mixture that became both food and physical support. After the organoids had reached a certain size, they were transferred into flasks that were kept in constant motion to keep the cells and tissues exposed to oxygen and nutrients.
After two months, the brain organoids stopped developing, probably because they lacked a blood supply to deliver oxygen and food deeper into the tissues, researchers said.
By studying the gene expression of the different tissues of the organoid, researchers were able to identify discrete brain areas including the dorsal cortex, prefrontal cortex, the forebrain and ventral forebrain, the hippocampus, choroid plexus and immature retina — the beginnings of the eye.
Not all of these areas developed in every organoid, however, and they didn’t look exactly like the brains of human embryos as they grow in the womb.
“In a developing embryo, you have the cerebral cortex at the front, then the ventral forebrain below that, and behind that you have the mid-brain, the cerebellum and the brain stem,” explained Madeline Lancaster, a postdoctoral researcher at the Institute of Molecular Biotechnology.
“In ours, we don’t have that spatial organization. We have those regions, but they’re not spatially organized in that manner,” she said.
And while researchers found some evidence that the different brain regions were functioning, they don’t think the organoids were fully wired and connected the way mature adult brains are, because that kind of connection is something that happens at later developmental stages.
“It’s sort of like manufacturing all the transistors and resistors in a radio, but not actually wiring it all up so you can listen to the radio,” said Amy Bernard, director of structured science for the Allen Institute for Brain Science in Seattle. “But certainly getting those building blocks set in is the first step.”
However, “it’s very impressive to see the level of differentiation that’s achieved in this model,” added Bernard, who was not involved with the study.
To further prove the value of watching early brain development this way, the researchers took stem cells from an individual with microcephaly, a developmental problem that affects about 25,000 of the roughly 4 million children born in the United States each year.
They treated the stem cells with chemicals to return them to an embryonic state and then watched them as they began to grow into an early brain.
Compared to the way previous organoids had grown, the stem cells from the individual with microcephaly stopped dividing earlier, so they had fewer total stem cells with which to build a brain, resulting in a smaller overall brain size.
“So in this, we understand how microcephaly developed in one individual patient,” Lancaster said.
Their hope is that by studying the process of microcephaly and other developmental problems in many individuals, they will find new ways to diagnose and perhaps treat these kinds of conditions.
More information
For more on brain development through childhood and adolescence, visit the U.S. National Institute of Mental Health.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










