- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
- Understanding the Connection Between Anxiety and Depression
- How Daily Prunes Can Influence Cholesterol and Inflammation
FDA OKs 2-Drug Combo Treatment for Advanced Melanoma
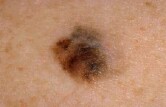
The drugs Mekinist and Tafinlar were approved for combination treatment of advanced melanoma skin cancer, the U.S. Food and Drug Administration announced Friday.
The two medicines “are the first drugs approved for combination treatment of melanoma,” Dr. Richard Pazdur, director of the Office of Hematology and Oncology Products in the FDA’s Center for Drug Evaluation and Research, said in an agency news release. “Their development for combination use is based on the strong understanding of the biological pathways of the disease,” he said.
The combined treatment was approved for use in patients with melanoma that cannot be removed by surgery or in people with late-stage melanoma that has spread. Both drugs were approved by the FDA last year as single treatments for these groups of patients.
Mekinist (trametinib) and Tafinlar (dabrafenib) block cellular signaling in different sites of the same molecular pathway that promotes cancer cell growth, the FDA explained.
The agency’s approval of the drugs for combination treatment of melanoma is based on the results of a clinical trial of 162 patients. It found that 76 percent of patients who received treatment with Mekinist and Tafinlar had their cancer shrink or disappear for an average of 10.5 months.
In comparison, 54 percent of patients treated with Tafinlar alone had their cancer shrink or disappear for an average of 5.6 months, the investigators found.
Clinical trials are underway to determine whether combination treatment with Mekinist and Tafinlar can boost patient survival, the FDA added in the news release.
The most common side effects reported by patients who received the dual treatment included fever, chills, tiredness, rash, nausea, vomiting, diarrhea, abdominal pain, swelling in the hands and feet, cough, headache, joint pain, night sweats, decreased appetite, constipation and muscle pain. Serious side effects included bleeding, blood clots, heart failure, skin and eye problems, and kidney damage.
Women of childbearing age should be advised that Mekinist and Tafinlar can cause birth defects, and women and men should also be told that treatment with Mekinist and Tafinlar may cause infertility, the FDA stated.
Melanoma is the most aggressive type of skin cancer and the leading cause of death from skin disease. It was estimated that 76,690 Americans would be diagnosed with melanoma and 9,480 would die from the disease in 2013, according to the U.S. National Cancer Institute.
Mekinist and Tafinlar are marketed by GlaxoSmithKline.
More information
The American Cancer Society has more about melanoma.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










