- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
- Understanding the Connection Between Anxiety and Depression
Entyvio Approved for Ulcerative Colitis and Crohn’s Disease
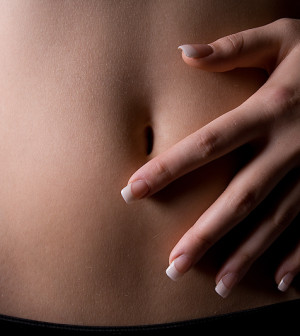

Entyvio (vedolizumab) has been approved by the U.S. Food and Drug Administration to treat adults with moderate-to-severe forms of two gastrointestinal conditions — ulcerative colitis and Crohn’s disease.
The approval applies to people for whom standard therapies — such as corticosteroids or tumor necrosis factor-blocking medications — have failed.
Ulcerative colitis, affecting about 620,000 Americans, causes inflammation and ulcers in the large intestine. This can lead to abdominal discomfort, bleeding and diarrhea, the FDA said in a news release.
Crohn’s causes inflammation and irritation of any part of the gastrointestinal tract. More than 500,000 Americans have been diagnosed with Crohn’s, the FDA said.
The most common side effects of Entyvio include headache, joint pain, nausea and fever. More serious adverse reactions observed during clinical testing included serious infection and allergy-like (hypersensitivity) reaction.
Though no clinical trial participants contracted a rare, often-fatal nervous system infection called progressive multifocal leukoencephalopathy (PML), the FDA said Entyvio users should be monitored for neurological signs of PML.
Entyvio is marketed by Takeda Pharmaceuticals America, based in Deerfield, Ill.
More information
The FDA has more about this approval.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










