- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
FDA Approves New Psoriasis Drug

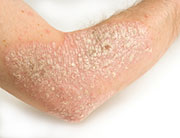
A new drug to treat adults with moderate-to-severe plaque psoriasis was approved Wednesday by the U.S. Food and Drug Administration.
People with plaque psoriasis, the most common form of the autoimmune skin disease, develop thick, red skin with flaky, silver-white patches called scales. In autoimmune diseases, the body’s immune system attacks healthy tissue by mistake.
The new drug, Cosentyx (secukinumab), is injected under the skin. The drug blocks a protein involved in the inflammatory response that causes plaque psoriasis, according to the FDA.
“Plaque psoriasis can cause significant skin irritation and discomfort for patients, so it is important to have a variety of treatment options available to patients,” Dr. Amy Egan, deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, said in an agency news release.
The FDA’s approval of the drug was based on four clinical trials that included more than 2,400 people and found that the drug was more effective than an inactive placebo. Cosentyx will carry information telling patients that because the drug affects the immune system, they may be at increased risk for infections.
One expert said the drug shows promise.
“The results seen from these clinical trials are increasing the efficacy bar compared to the data seen with previous psoriasis medications,” said Dr. Mark Lebwohl, chairman of the Kimberly and Eric J. Waldman Department of Dermatology at the Icahn School of Medicine at Mount Sinai, in New York City.
“Not only did record numbers of patients achieve 75 percent improvement,” Lebwohl said, “but large numbers of patients in the studies were 100 percent clear [after treatment].”
He added that the effectiveness of Cosentyx was better than any other long-term treatment for psoriasis.
However, doctors should be cautious when considering the use of Cosentyx in patients with a chronic infection or history of recurrent infection, and in patients with active Crohn’s disease, the FDA said.
Common side effects include upper respiratory infections and diarrhea.
The drug is intended for adults with plaque psoriasis who are candidates for drugs that travel through the bloodstream, phototherapy (ultraviolet light treatment) or both, the FDA said.
Dr. Doris Day, a dermatologist at Lenox Hill Hospital in New York City, said, “Now that we understand the biologic and genetic pathways that lead to psoriasis, we can better and more narrowly target those pathways to better control the condition in more precise ways.”
She added that “it’s very exciting to be able to offer patients greater choices for control of this chronic condition that not only affects the skin but often joints and other organ systems… as well as [having] a powerful negative impact on their self-esteem.”
Psoriasis is the most common autoimmune disease in the United States, affecting as many as 7.5 million Americans — about 2 percent of the population, according to the National Psoriasis Foundation.
Cosentyx is marketed by Novartis Pharmaceuticals Corp., of East Hanover, N.J.
More information
The American Academy of Family Physicians has more about psoriasis.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










