- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
Asthma Drug May Help Those With Chronic Hives

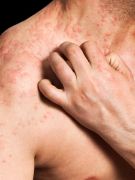
A drug already used to treat moderate-to-severe allergic asthma appears to offer relief to people with chronic hives who haven’t been helped by standard medications, new research suggests.
The prescription drug — omalizumab (Xolair) — is already available to treat hives, following U.S. Food and Drug Administration approval earlier this year for that use.
The current study confirms that when Xolair is taken at a high dose for a six-month period it seems to be both safe and effective at controlling the severe and often debilitating itching that characterizes long-term hives.
“So what we’re talking about here are only chronic cases, in which patients have hives that last for more than six weeks,” explained study senior author Dr. Karin Rosen, an associate group medical director with Genentech Inc., in San Francisco. “That’s usually just .5 to 1 percent of hives patients.”
“But for those patients, this is a really horrible disease,” she noted. “And until now antihistamines have been the only approved drug for chronic hives. But to work, antihistamines often need to be used at up to four times approved levels, which has a sedating effect. And 50 percent of the time patients didn’t respond anyway.”
“So then,” said Rosen, “they would try other types of medications, like systemic steroids. But over time steroids can have very severe side effects. So then we would turn to the heavy artillery, like [the immunosuppressant drug] cyclosporine, which also has very severe side effects, and can’t be taken by people with high blood pressure or heart disease.”
“All this means that many, many patients have simply been left without any treatment,” she noted. “But with this drug we are seeing great improvement in patients who previously had no options. And that’s very, very encouraging.”
Rosen and her colleagues discuss their findings in the July 21 issue of the Journal of Investigative Dermatology. The current study is the latest in a series of ongoing studies, which are being funded by drug manufacturer Genentech Inc. and Novartis Pharma AG in Basel Switzerland.
While not lethal, chronic hives can last for months, and tend to come and go without a clear idea of what brings it on. And, Rosen noted that almost half of chronic hive patients also have angioedema, a “scary and deforming” condition that involves deep tissue swelling, often affecting the eyes and the tongue.
To explore Xolair’s potential, the team tested its effectiveness among more than 260 chronic hives patients for whom prior treatment had failed.
Patients were randomly divided into four groups, in which they were respectively treated with a once-a-month injection of Xolair at either a 75 milligram (mg) dose, a 150 mg dose, a 300 mg dose, or a dummy shot.
Tracking was conducted for both a three-month period and a six-month period.
The team found that by the 12th week, people given Xolair showed a marked reduction in their overall number of hives, hive intensity and itching severity, compared with patients who got the dummy treatment. And Rosen noted that roughly 40 percent saw a complete disappearance of all symptoms, while 80 percent experienced “what we call minimally important improvement.”
Symptom relief was maintained through the six-month mark, and the authors determined that those treated with the highest (300 mg) dosage of Xolair saw the biggest improvement.
Side effects were described as being mild to moderate, and included headaches, joint pain, sinus infection and reactions at the point of injection.
The study authors acknowledged that more research will be needed to see how the drug performs over longer periods of time.
Dr. Robert Kirsner, chair of the department of dermatology and cutaneous surgery and chief of dermatology at the University of Miami Miller School of Medicine, suggested that the new intervention could prove to be a boon for a condition that “can significantly affect patients’ quality of life.”
“Patients suffer from this severe, recurrent itching, and it is a challenge for clinicians to find treatments that can help,” he noted. “The data related to omalizumab use, in a well-performed randomized clinical trial, is extremely promising and offers hope for patients who have chronic [hives], and for the physicians who care for them.”
More information
Visit the Asthma and Allergy Foundation of America for more on hives.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










