- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
FDA Approves Landmark Sickle Cell Gene Therapies
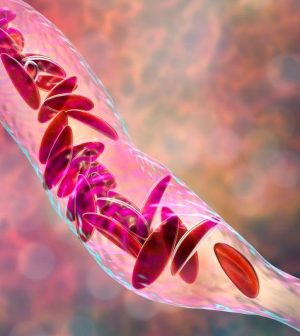
The U.S. Food and Drug Administration on Friday approved two milestone gene therapies for sickle cell disease, including the first treatment ever approved that uses gene-editing technology.
Casgevy, developed by Vertex Pharmaceuticals of Boston and CRISPR Therapeutics of Switzerland, is the first medicine available in the United States to treat a genetic disease using the CRISPR gene-editing technique.
The one-time treatment permanently changes DNA in a patient’s blood cells, freeing them from the excruciating symptoms of sickle cell disease for life. But the relief will likely be very expensive, experts say.
The other approved therapy, Lyfgenia, uses a common virus to deliver genetic modifications to a patient’s blood stem cells in their bone marrow. That therapy was developed by Bluebird Bio, of Somerville, Mass.
“Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need, and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,” Dr. Nicole Verdun, director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research, said in an FDA news release.
“Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited,” Verdun added.
In both therapies, stem cells are removed from a patient’s blood for treatment.
With Casgevy, CRISPR gene-editing technology knocks out a gene that triggers the development of defective, crescent-shaped blood cells. Meanwhile, medicine kills off flawed blood-producing cells in patients, who are then given back their own altered stem cells.
The same thing happens with Lygenia, only a virus is used to deliver a genetic payload that causes the blood cells to start producing healthy hemoglobin.
The genetically modified blood stem cells are then given back to the patient as a one-time, single-dose infusion.
“These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, noted in the FDA news release.
“Today’s actions follow rigorous evaluations of the scientific and clinical data needed to support approval, reflecting the FDA’s commitment to facilitating development of safe and effective treatments for conditions with severe impacts on human health,” Marks added.
Evidence supporting Casgevy’s approval involved 44 patients treated with the gene-editing process. Out of 31 patients with sufficient follow-up time to be evaluated, 29 had complete freedom from the severe pain and organ damage that can occur during a sickle cell exacerbation.
“Anything that can help relieve somebody with this condition of the pain and the multiple health complications is amazing,” Dr. Allison King, a professor at Washington University School of Medicine in St. Louis, told the Associated Press recently. “It’s horribly painful. Some people will say it’s like being stabbed all over.”
Further, no patients experienced graft failure or graft rejection.
Vertex has said it plans to follow clinical trial patients for 15 years.
Victoria Gray, who has received the gene therapy, shared her experience with researchers at a scientific conference recently, saying she felt she “was being reborn” when she got the therapy, the AP reported. Gray had experienced bouts of terrible pain since childhood.
Now, she is active with her kids and works full time.
“My children no longer have a fear of losing their mom to sickle cell disease,” she said.
Lyfgenia’s approval was supported by a 24-month study in which 32 patients received the therapy. Of those, 28 had no sickle cell events following their transplant.
Sickle cell disease affects the protein that carries oxygen in red blood cells. The cells can become crescent-shaped because of a genetic mutation. This can block blood flow and cause pain, organ damage and stroke. The disease affects millions of people around the world.
It occurs more often in places where malaria is common, like Africa and India. Being a carrier of the trait may protect against severe malaria.
Standard treatments include medications and blood transfusions. A bone marrow transplant from a closely matched donor without the disease is the only cure.
Prices for the two gene therapies haven’t been released.
However, a price tag of around $2 million would be considered cost-effective because the existing treatments cost about $1.6 million for women and $1.7 million for men from birth to age 65, according to recent research.
“But if you think about it,” King said, “how much is it worth for someone to feel better and not be in pain and not be in the hospital all the time.”
More information
The U.S. Centers for Disease Control and Prevention has more on sickle cell disease.
SOURCE: U.S. Food and Drug Administration, news release, Dec. 8, 2023; Associated Press
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










