- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
Osteoporosis Drugs’ Long-Term Use Needs More Research: FDA

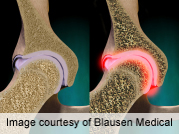
The long-term risks and benefits of taking bisphosphonates, a class of drugs widely used for osteoporosis, require more research, according to the U.S. Food and Drug Administration.
Bisphosphonates have been used in the United States since 1995 to treat people with osteoporosis, a condition in which bones become weak and are at increased risk of breaking. More than 44 million Americans are at risk for osteoporosis.
“These drugs clearly work. We just don’t know yet the optimum period of time individual patients should be on the drug to both maximize its effectiveness and minimize potential risks,” Dr. Marcea Whitaker, the review’s co-author and a medical officer at the FDA’s Center for Drug Evaluation and Research, said in an agency news release.
The FDA review of clinical studies assessed the effectiveness of long-term bisphosphonate use and concluded that some patients can stop using the drugs after three to five years and still continue to get their benefit.
But the review, which was published in 2012 in the New England Journal of Medicine, also called for more research into the drugs, sold under the brand names of Actonel, Atelvia, Boniva and Fosamax, along with generics.
Specifically, investigators need to learn more about patients’ fracture risk after they stop taking the drugs, and whether resuming them later could be helpful, Whitaker said.
The studies included in the review indicate that it may be prudent for patients with a low risk for fractures — younger patients who have near-normal bone density and no history of fractures, for example — to stop using bisphosphonates after three to five years.
However, continued use of bisphosphonates may benefit patients at increased risk for fractures, such as older patients with low bone density and a history of fractures.
There are a number of potential risks associated with bisphosphonates, including severe jaw bone decay and unusual thigh bone fractures. The FDA is currently examining a possible link between bisphosphonates and esophageal cancer.
Due to these potential risks, doctors may want to reconsider how long patients should take bisphosphonates, the FDA news release said.
If you’re taking bisphosphonates, talk to your doctor about whether or not you should continue taking the drugs, and re-evaluate the decision periodically, Whitaker said. Do not stop taking these drugs without first talking to your doctor.
Tell your doctor if you develop any new hip or thigh pain, or have any concerns about taking bisphosphonates. Report unusual side effects of bisphosphonates to the FDA’s MedWatch program.
More information
The National Osteoporosis Foundation has more about osteoporosis medicines.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










