- Comparing Whey and Plant-Based Protein: Which is Best?
- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
Used to Gauge COVID Severity, Pulse Oximeters Can Be Inaccurate on Darker Skin
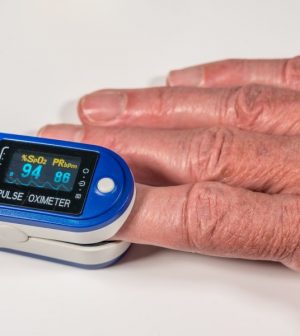
Pulse oximeters — small devices that clip onto fingertips — can seem like a handy way to gauge oxygen levels while monitoring a case of COVID-19. But people of color should be cautious about relying on them because they’re more likely to give inaccurate readings when used on darker skin, the U.S. Food and Drug Administration announced Friday.
The warning was based on a study published recently in the New England Journal of Medicine. It found Black patients had nearly three times the frequency of “occult hypoxemia” — low oxygen levels in the blood — as detected by “gold standard” blood gas measurements but not detected by pulse oximetry, when compared to white patients.
The FDA is now cautioning patients and health care providers that although widely used pulse oximeters can be useful, they also can be inaccurate.
“Although pulse oximetry is useful for estimating blood oxygen levels, pulse oximeters have limitations and a risk of inaccuracy under certain circumstances that should be considered,” the agency said in a statement.
Pulse oximeters use light beams to estimate oxygen saturation of the blood and pulse rate. As the fear of COVID-19 infection rose among Americans, many have rushed to buy the relatively cheap, over-the-counter devices to help determine if they need to go to the hospital when the illness strikes.
Oxygen saturation values are between 95% and 100% for most healthy individuals, but sometimes can be lower in people with lung problems, the FDA said. They also are generally slightly lower for those living at higher altitudes.
Any level in the low 90s is considered the threshold for illness that may need hospital care.
Still, “these devices are not intended to be the sole or primary use of information to make a clinical diagnosis or treatment decision,” Dr. William Maisel, director of the Office of Product Evaluation and Quality at the FDA’s Center for Devices and Radiological Health, told CNN in an interview. “Someone shouldn’t overly rely on a pulse oximeter reading, even if it’s the most accurate product.”
And although in many cases the level of inaccuracy may be small, there is a risk that an inaccurate measurement could result in unrecognized low oxygen saturation levels, the FDA said. That could keep a person who truly needs hospital care from reaching out.
Not a new finding
The NEJM study involved more than 10,000 patients and was led by Dr. Michael Sjoding at the University of Michigan. It found that in White patients, the pulse oximeter gave a misleading number 3.6% of the time, but in Black patients inaccuracies occurred 11.7% of the time. The reason: Because the devices rely on light, skin tones can alter the results.
Studies reaching back to the early 2000s have found similar race-based discrepancies with pulse oximetry, but many doctors may not be aware, Sjoding said.
Speaking with CNN, he said, “I am a pulmonary and critical care physician. One of our coauthors is a prominent Black physician from the University of Michigan. None of us knew this. None of us knew about these studies from the mid 2000s. It was not a part of our training.”
The FDA reviews prescription pulse oximeters, which are most often used in hospitals and doctors’ offices, but not over-the-counter oximeters sold directly to consumers for general wellness or sports/aviation.
Though the over-the-counter devices are not intended for medical purposes, some people use them to monitor COVID-19 at home.
The advisory suggests that patients who are monitoring conditions such as COVID-19 at home should pay attention to all their symptoms and not only blood oxygen readings. Not only skin pigmentation can affect a pulse oximeter reading, but also skin thickness, poor circulation, skin temperature, current tobacco use and even use of nail polish.
What to do?
According to the FDA, to get the best reading when using a pulse oximeter, remove any nail polish and make sure your hand is warm, relaxed and held below your heart level. Sit still. Wait a few seconds until the reading displays a steady number.
Pay attention to whether the oxygen level is lower than earlier measurements or is decreasing over time. Changes or trends in measurements may be more meaningful than one single measurement.
Not everyone who has low oxygen levels shows all or even any of the typical signs, but most common are bluish coloring in the face, lips or nails; shortness of breath or difficulty breathing; a cough that gets worse; restlessness; discomfort; chest pain or tightness, and fast or racing pulse rate.
It’s important to contact your health care provider if you’re concerned about your pulse oximeter readings or other symptoms. Also contact your doctor or your local health department if you think you may have COVID-19, so you can get a diagnostic test.
The FDA advised doctors to be aware of the limitations as well. Different brands of pulse oximeters and even different sensors may have varying accuracy levels, the FDA said. Pulse oximeters are least accurate when oxygen saturation is less than 80%.
Consider accuracy limitations when using the pulse oximeter to assist in diagnosis and treatment decisions. Use pulse oximeter readings only as an estimate of blood oxygen saturation, the FDA advised. For example, a pulse oximeter saturation of 90% may represent an arterial blood saturation of 86% to 94%.
The pandemic may finally be getting the FDA to better safeguard pulse oximeters. According to CNN, a group of U.S. senators sent the FDA a letter late last month urging them to investigate the issue.
Right now, the agency requires pulse oximeters to be tested on a variety of skin tones, meaning “at least two darkly pigmented [test subjects] or 15 % of the subject pool, whichever is larger,” CNN said.
More information
The American Lung Association offers some additional cautions for using a pulse oximeter.
SOURCES: U.S. Food and Drug Administration, news release, Feb. 19, 2021; CNN
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










