- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
FDA Panel Tackles Faulty Pulse Oximeter Readings That Come With Darker Skin
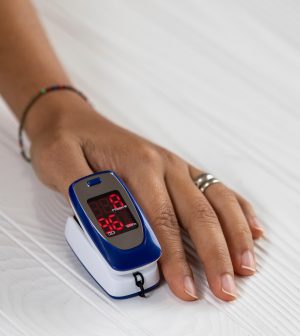
A U.S. Food and Drug Administration advisory panel on Friday addressed a continuing issue with pulse oximeters — that they give less accurate readings for folks with darker skin.
The devices are designed to measure oxygen levels in the blood, so correct readings are critical, experts say.
During its daylong meeting, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel reviewed ways to better evaluate the accuracy of pulse oximeters in patients with darker skin.
Although there is more work to be done when it comes to making pulse oximeters more accurate, panel member Dr. Jeffrey Feldman said the devices’ benefits of these devices still outweigh their limitations.
“This technology has and continues to save lives on a daily basis in this country. … It needs to be improved. We need to look at health disparities, and we need to do better,” he said after the meeting, CNN reported. “But we also need to recognize how valuable this technology is for patients every day, at home and in the hospital.”
Precisely because the general public can use these devices at home to check their oxygen levels, the panel honed in on how to ensure the accuracy of pulse oximeters for all skin tones before they reach drugstore shelves.
So, the panel focused on the structure of company trials testing the products.
Back in 2013, the FDA issued premarket guidance for developers of pulse oximeters, recommending that they have “a range of skin pigmentation” represented in their clinical studies of the devices, including at least two “darkly pigmented subjects or 15% of the study group,” whichever is larger.
Now, the FDA is weighing proposals to update these clinical trials to include more diverse groups of people, with at least 24 participants spanning the entire range of skin tones on what is known as the 10-shade Monk Skin Tone scale.
“There’s no question that more diversity needs to be a part of whatever new requirements that they would issue,” Feldman said.
“The prior requirements in 2013 were small numbers and really not very diverse — the only requirement was for up to two patients of color — and so that I think has proven to be inadequate to predict real-world performance,” he added.
Many of the panel members agreed that even 24 participants spanning different skin tones may not be a large enough sample size.
“Twenty-four patients just seems low to me,” member Dr. Thomas Wiswell, a neonatologist from Hawaii, said during the meeting, CNN reported.
“With 24, I still don’t know what that power is going to look like. My hunch is, it’s probably insufficient,” said Dr. Ben Saville, a member of the panel and a biostatistician in Texas.
Still, the panel mostly agreed that using the Monk Skin Tone scale would be better than what is used now.
“It’s the most diverse scale that we’ve discussed today,” member Dr. Cheryl Gooden, an associate professor of anesthesiology and pediatrics at the Yale School of Medicine, said during the meeting, CNN reported.
Edward McClure, who was diagnosed in 2013 with emphysema, said he relies on pulse oximeter devices to check his blood oxygen levels because he has been prescribed oxygen therapy. But his readings have not always been accurate, possibly due to his skin color.
“Sometimes, when I get a reading, the pulse rate might say 27, and the OT rating might say 90 — and then I know that is not correct, because I know my pulse was not 27,” McClure, who works with the nonprofit Right2Breathe, told the panel.
“Then I’ll do it again, and it might be closer to what I feel is right, but sometimes I have to do it up to three times where I’m convinced that this is the right reading,” he added. “These pulse oximeters don’t always read accurately for people who have melanated skin or heavily melanated skin like myself.”
Past research backs that up.
“Studies have shown that pulse oximeters are three times more likely to provide misleading readings for patients with darker skin pigmentations, leading to missed critical diagnoses of low blood oxygen levels,” Dr. Jesse Ehrenfeld, president of the American Medical Association, told the panel, CNN reported.
One 2022 study on flawed pulse oximeter readings found that among more than 3,000 hospitalized patients receiving intensive care, those who were Asian, Black and Hispanic received less supplemental oxygen than White patients, and that was linked to differences in their pulse oximeter readings.
Another study published in 2022 found that Black patients had higher odds than White patients of having low blood oxygen noted in their blood-drawn readings that weren’t detected by pulse oximetry.
Those disparities became even more pronounced during the pandemic
“We know that Black and brown people were disproportionately affected by the pandemic, with Black people accounting for a larger percentage of morbidity and mortality,” Dr. Dionne Ibekie, an anesthesiologist in central Illinois who also specializes in racial disparities in medicine, told CNN.
“The driving assumption was that this was due mainly because of social determinants of health such as environmental racism, lack of access to care, and lower employment status to name a few,” she said. “Social determinants absolutely do have an impact on health outcomes in Black and brown populations, including with COVID-19. However, the pandemic made the medical community look a little further into the cause of disparity in outcomes and focus more on the bias of the pulse ox.”
More information
Visit the U.S. Food and Drug Administration for more on pulse oximeters.
SOURCE: U.S. Food and Drug Administration, advisory committee meeting materials, Feb. 2, 2024; CNN
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










