- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
Experimental Drug Helps Body Fight Advanced Melanoma: Study

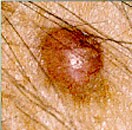
An experimental drug that harnesses the power of the body’s immune system to fight cancer has helped some patients with advanced melanoma keep their disease in check for several years, a new study indicates.
Researchers think the drug, which is called nivolumab, may help reset the immune system so that as a tumor adds new cells, the immune system is able to clear them away.
“We’re hypothesizing that we have reset the balance between the immune system and the tumor so there’s a state of equilibrium or co-existence. And that situation can go on for quite a long time, and we don’t really know how long it can go on,” said study author Dr. Suzanne Topalian, director of the melanoma program at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University in Baltimore.
An expert who was not involved in the study called the results “remarkable.”
“This really offers patients tremendous amounts of hope. We’re talking about potentially lengthening patients’ survival to the point that they might not die of their disease at all,” said Dr. Anthony Olszanski, co-director of the Medical Oncology Melanoma Program at Fox Chase Cancer Center, in Philadelphia.
The finding is part of the latest round of results of an early trial of nivolumab, which was tested in 107 patients. The patients enrolled in the study between 2008 and 2012.
Some tumors make molecules that switch off the immune system’s ability to recognize and kill cancer cells. Nivolumab essentially releases the brakes a cancer has applied to immune attack cells, restoring the body’s ability to clear the disease.
All of the patients in the study had the dangerous skin cancer known as advanced melanoma, and 80 percent had tumors that had spread to other organs. More than half of patients had tried at least two previous treatments without success.
People in the study received intravenous doses of the drug every two weeks for up to two years. Patients stopped treatment if their tumors disappeared, the side effects became too toxic, their tumors continued to grow or they withdrew their consent to participate in the study.
About half of patients who took the drug reported side effects. The most common were fatigue, rash and diarrhea. Serious adverse events were seen in five patients. Most side effects occurred within the first six months of treatment.
Thirty-three patients who took the drug saw their tumors shrink by at least 50 percent on the medication. In a few cases, the masses disappeared completely.
Another seven patients had stable disease, meaning their cancer didn’t get worse or better for at least six months.
The other 67 patients had mixed responses — meaning some of their tumors grew while others shrank, or their tumors grew despite treatment.
Overall, 62 percent of patients who enrolled in the trial were still alive a year later, and 43 percent were still alive after two years.
As of March 2013, the last study update, researchers reported 19 patients were still alive. A few have maintained their results for more than a year after taking their last dose of the drug.
In contrast, studies of another immune therapy for melanoma, a drug called Yervoy, found that up to 49 percent of patients were still alive after one year and up to 33 percent of patients were still alive two years after taking the medication. Yervoy was approved by the U.S. Food and Drug Administration in 2011.
“The fact that this article quoted one-year and two-year survival rates that are as high as they are is truly outstanding,” said Olszanski.
“These results, if they are duplicated in a large study, they will be practice changing for patients with melanoma and this will quickly vault to a first-line therapy,” he said.
Researchers said larger studies of nivolumab were already underway.
The findings were reported online March 3 in the Journal of Clinical Oncology.
The study was sponsored by drug makers Bristol-Myers Squibb and Ono Pharmaceutical. Several study authors reported financial ties to the company. The researchers would not speculate about the potential price of nivolumab, but drugs of this ilk tend to cost thousands of dollars a month.
More information
Visit the American Cancer Society to learn more about immunotherapy for treating melanoma.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










