- Comparing Whey and Plant-Based Protein: Which is Best?
- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
Immune-Based Treatment May Fight Advanced Cervical Cancer
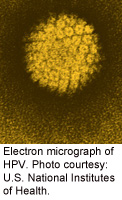
A new type of therapy shows promise in treating some women with advanced cervical cancer, researchers say.
The majority of cervical cancers are caused by the human papillomavirus (HPV). This new treatment — called HPV-targeted adoptive T cell therapy — boosts the body’s natural immune response to HPV in cervical cancer tumors, the study authors explained.
First, HPV-targeted T cells — immune cells that specifically attack tumor cells that contain HPV proteins — are grown from a patient’s tumor in the laboratory. The T cells are then put back into the patient’s body to fight her cancer.
In this U.S. National Cancer Institute-supported study, nine women with advanced cervical cancer underwent the therapy, and three responded to it. One of those three patients had a 39 percent reduction in tumor volume, while the other two had complete remissions that had lasted for 11 months and 18 months by the time the study was written.
“This proof-of-principle study shows that adoptive transfer of HPV-targeted T cells can cause complete remission of metastatic cervical cancer and that this remission can be long-lasting,” lead author Dr. Christian Hinrichs, an assistant clinical investigator at the U.S. National Cancer Institute, said in a news release from the American Society of Clinical Oncology (ASCO).
The therapy was linked to serious side effects, however, including low blood counts, infections and metabolic disorders, the researchers said. Hinrichs and colleagues were scheduled to present the findings Monday in Chicago at the ASCO annual meeting.
Adoptive T cell therapy has previously shown promise in treating melanoma, leukemia and sarcoma, but this is the first time it has been tested in cervical cancer patients.
“Cellular therapy might have application to a broader range of tumor types than previously recognized,” Hinrichs said. However, he added, “this treatment is still considered experimental and is associated with significant side effects. We also need to explore why this therapy worked so well in certain women, and not in others.”
Studies presented at medical meetings are typically considered preliminary until published in a peer-reviewed journal, but two experts in the field remained cautiously optimistic about the new therapy.
“I think that this small pilot study provides an important proof-of-concept,” said Dr. Louis Weiner, director of the Georgetown Lombardi Comprehensive Cancer Center in Washington, D.C.
“Engineered T cells can identify and destroy metastatic cancer cells when those cells express a truly cancer-relevant target,” Weiner said. “While the field is still young, and this study is preliminary, these results provide optimism that people’s T cells can be reprogrammed to treat many types of cancer.”
Dr. Krzysztof Misiukiewicz, assistant professor of medical oncology at the Icahn School of Medicine at Mount Sinai in New York City, agreed. He said the research is a step forward in the treatment of cancers linked to HPV, but added that more studies are needed to help bring “regulatory approval of adoptive T cell therapy as a standard of care.”
Misiukiewicz also stressed that more work must be done to help predict which patients will benefit most from the treatment, to shorten the time needed to culture the necessary T cells in the laboratory, and to boost the “memory” of the T cells so they can best fight tumor cells.
In the ASCO news release, expert oncologist Dr. Don Dizon added: “These preliminary data demonstrate, not only the viability of this approach, but that gains in survival can be realized in a cancer where patients have little to no effective treatment options and where median survival is usually less than two years.”
More information
The U.S. National Cancer Institute has more about cervical cancer.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










