- Comparing Whey and Plant-Based Protein: Which is Best?
- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
Immune-Focused Drugs Show Promise Against Melanoma
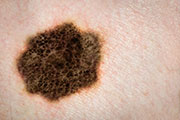
Drugs that supercharge the body’s immune system show promise in treating advanced melanoma, according to a pair of clinical trials.
The trials both involve drugs called immune checkpoint inhibitors, which essentially prod the immune system to attack and destroy cancer cells, said Dr. Suzanne Topalian, director of the Melanoma Program at Johns Hopkins’ Sidney Kimmel Comprehensive Cancer Center in Baltimore.
In one trial, researchers found that an immune checkpoint inhibitor called Keytruda (pembrolizumab) outperformed the current frontline treatment for advanced melanoma, another immune-boosting drug called Yervoy (ipilimumab).
The other trial showed that patients responded better to a combination of two different types of immune checkpoint inhibitors than to Yervoy used on its own.
Both trials were slated for presentation Monday in Philadelphia at the annual meeting of the American Association for Cancer Research, and both were also simultaneously published in the New England Journal of Medicine.
The new drugs provide hope for patients with advanced melanoma, which up to now has proven a swift and fatal disease, said Dr. Gary Schwartz, chief of the division of Hematology/Oncology at Columbia University Medical Center in New York City.
“We were lucky if patients lived nine to 11 months,” said Schwartz, who also is an expert for the American Society of Clinical Oncology. “Now we have patients living five or 10 years with metastatic disease, and that was unheard of in melanoma.”
Schwartz said the immune-boosting drugs now being tested have the potential to help curb many other forms of cancer.
“The potential here is really limitless with immune activation. Now we can effectively turn on these immune switches and kill cancer cells,” he said. “This is the beginning of a new age of oncology, and it is starting with melanoma.”
The U.S. Food and Drug Administration approved Yervoy in 2011. The drug targets an immune system “switch” called CTLA4, which acts to rein in the body’s immune cells so they don’t run amok.
Cancers normally take advantage of controls like CTLA4 to avoid detection and destruction by the immune system. “It turns out these immune checkpoint molecules help the tumor create a shield around itself, preventing the immune cells from destroying it,” Topalian said.
The FDA subsequently has approved a second-generation set of immune checkpoint inhibitors that target a more cancer-specific “switch” called PD1, Schwartz said. These drugs currently are used if patients do not respond to Yervoy.
One of the anti-PD1 drugs, Keytruda, showed better results with fewer side effects in a phase III trial that compared it against Yervoy, researchers reported Monday.
The trial involved 834 patients with advanced melanoma in 16 countries. Two-thirds received Keytruda, while the rest received the current front-line treatment.
At six months after treatment, progression-free survival — meaning the cancer had not grown — was about 46 percent for Keytruda and 26 percent for Yervoy. Overall survival rates after one year stood at 74 percent and 68 percent for Keytruda, depending on the dose patients received, compared with 58 percent for Yervoy.
About 33 percent of patients responded to treatment with Keytruda, compared with 12 percent for Yervoy, the study found.
Furthermore, only 12 percent of patients taking Keytruda suffered from side effects, compared with 20 percent in those who received Yervoy.
Keytruda performs better because it focuses on a switch involved in immune system cells that already have identified the cancer and are trying to attack it, said senior investigator Dr. Antoni Ribas, director of the Tumor Immunology Program Area at UCLA Jonsson Comprehensive Cancer Center.
The other clinical trial showed that combining two types of immune checkpoint inhibitors produced a good response in many more patients, even those with a genetic mutation that increases the ferocity of their melanoma.
However, the combination also greatly increased the risk of side effects, the researchers noted.
The trial involved 142 patients with advanced melanoma. Two-thirds received the combo therapy, which included the anti-CTLA4 drug Yervoy and the anti-PD1 drug Opdivo (nivolumab). Another third of patients received Yervoy alone.
The study found that in patients without the dangerous mutation, 61 percent responded to the combo treatment versus just 11 percent who responded to treatment with Yervoy alone.
Patients with the dangerous mutation also responded well to the combination therapy, with 44 percent receiving some benefit.
“We now have a combination of medicines that seem to benefit more than half of patients with malignant melanoma, regardless of mutation status,” said senior clinical trial investigator Dr. Jedd Wolchok, chief of Melanoma and Immunotherapeutics Service at Memorial Sloan Kettering Cancer Center in New York City.
However, about half of the patients receiving the combination therapy did suffer from moderate to serious side effects, compared with just a quarter of patients treated with Yervoy alone, the team found.
By unleashing the immune system to attack cancer, doctors run the risk that the immune cells also will start targeting the major organs of the body, Schwartz said. This can cause temporary organ damage and, in extreme cases, organ failure.
“This is not penicillin,” he said. “There are still times when the immune system will run amok, and you have to put it back in the box.”
Topalian noted that researchers in the studies were able to control the side effects by suspending the drug therapy and treating patients with inflammation-dampening steroid medications.
But Dr. Len Lichtenfeld, deputy chief medical officer for the American Cancer Society, said the seriousness of these side effects cannot be ignored.
“We understand better now how to manage some of those side effects, but the reality is they can be significant and they require a team of professionals working together to manage those side effects,” Lichtenfeld said. “It is not a simple treatment.”
The new cancer drugs are not cheap, either. Topalian said treatment with any of them will easily top $100,000 a year.
That said, both clinical trials represent significant advances in “a continuing evolution of our understanding how to treat patients with advanced melanoma,” Lichtenfeld said.
“Before, we had virtually nothing for the vast majority of advanced melanoma patients that would allow them to live more months,” Lichtenfeld said. “Now we have drugs where that is actually happening. For some patients, they’ve had responses where the disease has even stabilized or gone away, and they maintain those responses for a long period of time.”
The Keytruda trial was funded by the drug company Merck, while the combination therapy trial was funded by Bristol-Myers Squibb.
More information
For more on melanoma, visit the U.S. National Institutes of Health.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










