- Comparing Whey and Plant-Based Protein: Which is Best?
- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
Melanoma Drug Trials Show Significant Promise
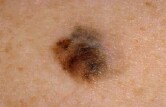
A relatively new drug appears effective in boosting survival for patients battling advanced melanoma, the deadliest form of skin cancer, according to a pair of preliminary studies.
The drug Yervoy (ipilimumab) “takes the brakes off the immune system,” improving the body’s ability to target and attack melanoma, said Dr. Philip Friedlander, assistant professor of dermatology at Mount Sinai’s Icahn School of Medicine in New York City.
The two trials report that Yervoy can dramatically extend survival for patients with stage 3 and stage 4 melanoma, adding months and years to their lives both on its own and in combination with another cancer drug.
“It’s a major breakthrough,” Friedlander said of the medication, which the Food and Drug Administration approved in 2011. “It’s a novel immune approach that’s harnessing one’s own immune system to fight cancer.”
The results of both trials are scheduled for presentation Monday at the American Society for Clinical Oncology’s annual meeting in Chicago. As such, the data and conclusions should be considered preliminary until published in a peer-reviewed medical journal.
The five-year survival rate for someone with stage 4 melanoma — cancer that has spread to other parts of the body — now stands at 15 percent to 20 percent, according to the American Cancer Society.
Yervoy works by blocking a receptor called CTLA-4 that normally deactivates the body’s lymphocytes, which are white blood cells that form the leading edge of an immune system response. By blocking CTLA-4, the drug unleashes the immune system to attack cancer cells.
In one of these trials, doctors extended the median survival of 53 patients with inoperable advanced melanoma by roughly three and a half years, said presenting study author Dr. Mario Sznol, a professor of medical oncology at Yale School of Medicine. They did this by combining Yervoy with another immune system drug called nivolumab.
The combination therapy nearly doubled the overall survival found in previous studies of either drug alone, the researchers reported.
Nivolumab, which is not yet approved by the FDA, works by disarming the tumor’s defense against attacks from the immune system, said Sznol.
“You’re really hitting two molecules that control immune activation at different locations,” Sznol said. “There are multiple ways that lymphocytes are inhibited. Maybe if you hit two of those mechanisms you get better results than if you just hit one.”
Sznol said he’s “never seen anything quite like” the results from using the two drugs in combination.
“If you look at all 53 patients we’ve treated, the one-year survival is 85 percent and the two-year overall survival is 79 percent,” he said. “It’s hard to compare across studies, because it’s a small study, but no matter how we select the patients, I’ve never seen a trial that had even close to a two-year survival of 79 percent.”
The results are even more impressive considering that many of the patients already had undergone as many as three rounds of drug therapy for their melanoma, said Dr. Len Lichtenfeld, deputy chief medical officer for the American Cancer Society.
“Many of those patients were pretreated before they got this drug, which means earlier treatment had failed,” Lichtenfeld said. “When you see results like that, you certainly stand up and take notice.”
Phase 3, or final, trials have been completed regarding the combination therapy, Sznol said, and it’s hoped the results will be out in a year.
The second clinical trial, which involved 951 patients, also reported strong results from Yervoy. It found that the drug reduced cancer’s return by roughly 25 percent when compared to placebo, or dummy, medication in patients who had surgery for high-risk stage 3 melanoma.
The drug was given every three weeks for four doses, and treatment continued at three-month intervals up to three years. Three-year, recurrence-free survival rates were 46.5 percent in the Yervoy group, compared with 34.8 percent in the placebo group.
Both studies reported severe side effects, however. Five treatment-related deaths occurred in the second clinical trial, and about half the patients discontinued treatment because of side effects such as skin rash and inflammation of the colon, thyroid and pituitary gland.
Side effects appeared even more pronounced in the combination therapy trial. “When we give the two drugs together, we see a greater incidence of side effects,” Sznol said.
However, he argued that the survival benefits and the high cost of Yervoy outweigh the risks. “I have a lot of confidence in medical oncologists and their ability to manage side effects from treatment,” Sznol said. “If later trials prove the efficacy is high enough, then the risk/benefit ratio is well worth it.”
Drug manufacturer Bristol-Myers Squibb prices Yervoy at $30,000 per injection, which means a full four-dose course of therapy will cost $120,000.
“Whatever the cost might be, if you’re getting this kind of efficacy, it might be a more cost-effective treatment than something that is less costly but much less effective,” Sznol said.
Bristol-Myers Squibb helped fund both studies, the researchers report.
A third clinical trial presented at the meeting found that another new immunotherapy drug called MK-3475 also provided significant survival benefits to advanced melanoma patients.
MK-3475 targets the same receptor as nivolumab, one of the drugs used in the combination therapy trial.
More than 400 patients took part in this phase 1 trial, an early effort in the approval process. One-year survival rates were 74 percent in patients not previously treated with Yervoy, and 65 percent in people who had previously received Yervoy for their melanoma.
The FDA has granted MK-3475 a priority review designation, meaning the agency wants to speed up the application review. This trial was funded by the drug’s maker, Merck.
More information
For more information on melanoma, visit the U.S. National Cancer Institute.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










