- Comparing Whey and Plant-Based Protein: Which is Best?
- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
Certain Abbott Blood Sugar Monitors May Give Incorrect Readings
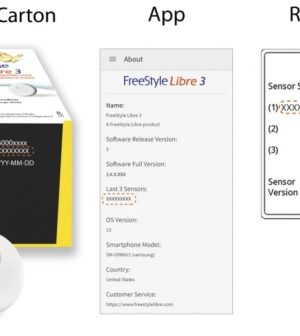
Abbott has warned diabetes patients that some of its continuous blood sugar monitoring systems may need to be replaced because of inaccurate readings.
“Abbott has recently identified a small number of FreeStyle Libre 3 sensors that may provide incorrect high glucose readings, which if undetected may pose a potential health risk for people living with diabetes,” the company said in an alert issued Thursday.
An inaccurate high blood sugar reading may prompt diabetes patients to take insulin when they don’t need it, the company said. That can trigger hypoglycemia (low blood sugar), a condition that can become life-threatening if not recognized or addressed, according to the Mayo Clinic.
Less than 1% of American users of the Libre 3 sensors are affected, with the affected devices being distributed in the first half of May, the company said.
Abbott added that it will replace the sensors at no charge. The company said people should check the company’s website to confirm whether their sensor is affected and then notify the company so a replacement sensor can be sent to their home. The sensors came from these three lot numbers, according to Abbott: T60001948, T60001966, T60001969.
Any adverse reactions with the use of these sensors should be reported to Abbott’s customer service line at 1-833-815-4273, the company said.
Such reports may also be sent to the FDA’s MedWatch Adverse Event Reporting program by completing Form FDA 3500 online at www.FDA.gov, calling 1-800-FDA-1088 or faxing to 1-800-FDA-0178, Abbott added.
A continuous glucose monitoring system uses a sensor, a reader and an app to help people with diabetes check their blood sugar without having to draw blood from their fingers. The U.S. Food and Drug Administration first approved the Abbott devices in 2017.
More information
The National Institute of Diabetes and Digestive and Kidney Diseases has more on glucose monitoring systems.
SOURCES: Abbott, news release, July 24, 2024
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










