- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
Eylea Approval Expanded to Include Diabetic Retinopathy
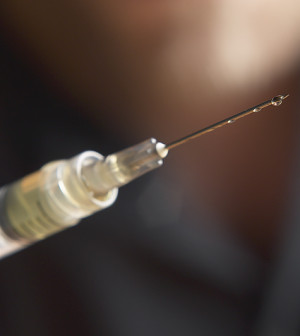

U.S. Food and Drug Administration approval of Eylea (aflibercept) has been expanded to treat diabetic retinopathy among people with diabetic macular edema, the agency said Wednesday in a news release.
Affecting more than 29 million people in the U.S., diabetic retinopathy is the most common diabetic eye disease and a leading cause of blindness, the FDA said. Among some people who have vision-impairing diabetic macular edema, diabetic retinopathy can spur the growth of abnormal blood vessels on the eye’s retina. If these vessels burst, it can lead to severe vision loss or blindness.
The injected drug Eylea is intended to be used in tandem with appropriate therapies to control blood sugar, blood pressure and cholesterol, the FDA said.
The drug’s safety and effectiveness were evaluated in clinical studies involving nearly 680 people. The most common side effects included bleeding of eye tissue called the conjunctiva, eye pain, cataracts, visual artifacts called floaters and increased eye pressure.
Eylea was first approved in 2012 to treat the eye condition wet age-related macular degeneration, and also was sanctioned to treat two conditions that cause fluid to leak into the eye’s macula, leading to blurred vision.
The drug is marketed by Regeneron Pharmaceuticals, based in Tarrytown, N.Y.
More information
The FDA has more about this approval.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










