- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
Opioid Addiction Treatment Rates in U.S. Have Flatlined, Study Finds
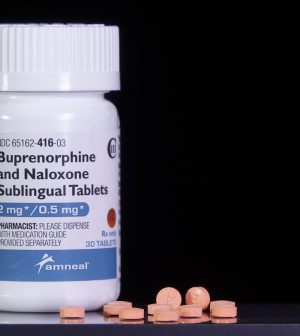
The U.S. opioid crisis led to changes that make it easier for people struggling with addiction to get medication from a health care provider to help them quit.
But researchers found that for some reason, rates of medication use haven’t budged.
Numbers of Americans who started buprenorphine were flat between 2019 and 2022, after rising from January 2016 to September 2018.
Those who stayed on the medication for at least six months hovered around 20% from 2016 to 2022. Staying on the medication for longer is associated with reduced risk of opioid overdose death.
Researchers from the University of Michigan and Boston University used national prescription dispensing data to study medication trends.
The changes that could have increased buprenorphine use included one in spring 2020 that allowed providers to see patients via telehealth, instead of requiring an in-person visit before prescribing buprenorphine.
Then, in spring 2021, prescribers no longer needed to meet an eight-hour federal education requirement to issue the medication.
“The fact that buprenorphine initiation and retention did not rise after these efforts were implemented suggests that these policy changes were insufficient to address the barriers to prescribing enough to meet the rising need for this medication,” said lead study author Dr. Kao-Ping Chua, an assistant professor of pediatrics in the Susan B. Meister Child Health and Evaluation Research Center at Michigan.
In 2023, prescribers including doctors and nurse practitioners who are already allowed to prescribe other controlled substances can now prescribe buprenorphine without needing special approval from the federal government.
“It remains to be seen whether the elimination of the waiver requirement will move the needle on buprenorphine initiation and retention,” Chua said in a Michigan news release. “Based on our study, it seems likely that this intervention will be insufficient to overcome the many other barriers to buprenorphine initiation and retention, such as stigma about the drug among clinicians, patients and pharmacists.”
That part may not get easier. The U.S. Drug Enforcement Agency has proposed partially reversing the change that allowed telehealth instead of in-person visits, he said.
All clinicians also must now complete eight hours of training in addiction treatment when they renew their controlled substance license.
“I worry that this requirement, which hasn’t been well-publicized, could result in some prescribers no longer having a controlled substance license entirely, decreasing the number of eligible prescribers,” said senior study author Amy Bohnert, a professor in Michigan’s Department of Anesthesiology.
Earlier research by Chua and colleagues found that only 1 in 12 patients who were seen in an emergency department for an opioid overdose were prescribed buprenorphine within 30 days. By comparison, about half of patients seen by emergency doctors for a severe allergic reaction called anaphylaxis received a prescription for epinephrine.
“My hope is that our new study will further underscore how much the health care system is underusing a critical tool to prevent opioid overdose deaths,” Chua said.
The findings were published as a research letter in the April 25 issue of the Journal of the American Medical Association.
One limitation of the study is that the data came exclusively from retail pharmacies and not from doctor visits or in treatment programs.
More information
The U.S. Centers for Disease Control and Prevention has more on medications for opioid use disorder.
SOURCE: University of Michigan – Michigan Medicine, news release, April 25, 2023
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










