- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
Journal Publisher Retracts Two Studies Cited in Abortion Pill Access Case
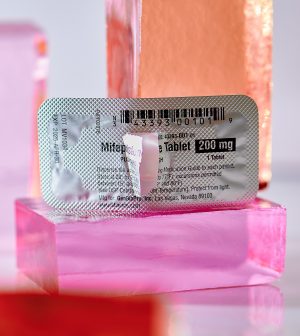
Two studies that warned of the harms of the abortion pill have been retracted by the journals’ publisher over flaws in the data and conflicts of interest among the researchers.
Complicating matters even further, the papers were cited in a Texas Court ruling that has challenged nationwide access to the abortion pill. That landmark case is headed to the U.S. Supreme Court next month, with the high court’s ruling expected to determine access to the abortion pill across the country.
Demand for the abortion pills mifepristone and misoprostol has surged as states have imposed abortion bans or restrictions following the Supreme Court’s reversal of Roe v. Wade in June 2022. Medication abortion now accounts for more than half of all abortions in the United States and typically involves two drugs, mifepristone and misoprostol, the Associated Press reported.
In its retraction, publisher Sage Perspectives said “we made this decision with the journal’s editor because of undeclared conflicts of interest and after expert reviewers found that the studies demonstrate a lack of scientific rigor that invalidates or renders unreliable the authors’ conclusions.”
The studies cited in the Texas court ruling were published in the journals Health Services Research and Managerial Epidemiology. They were both supported by the Charlotte Lozier Institute, which seeks to end access to abortion.
A 2021 paper looked at 423,000 abortions and more than 121,000 emergency room visits following medication abortions and abortions done using a medical procedure from 1999 to 2015. The conclusion? Medication abortions are “consistently and progressively associated with more post-abortion ER visit morbidity [illness]” than those performed during a medical procedure.
Meanwhile, a 2022 paper concluded that failure to identify a prior abortion during an ER visit — either by a doctor or because a patient concealed it — is “a significant risk factor for a subsequent hospital admission.”
In the Texas court ruling, U.S. District Judge Matthew Kacsmaryk argued that mifepristone’s approval by the U.S. Food and Drug Administration in 2000 was flawed because it overlooked serious safety issues with the pill.
He cited one of the retracted studies in claiming that mifepristone causes “many intense side effects.” His ruling also cited the second retracted paper in explaining why anti-abortion physicians had the legal standing to bring their lawsuit, because medication abortions cause “enormous pressure and stress” to physicians.
A federal appeals court overturned parts of the Texas ruling last summer, but the Supreme Court will rule definitively on just how accessible medication abortions should be.
James Studnicki, lead author of both studies, told the AP that the publisher’s actions are a “baseless attack on our scientific research and studies.” Studnicki is a vice president at the Charlotte Lozier Institute.
More information
Yale Medicine has more on medication abortions.
SOURCE: Sage Perspectives, news release, Feb. 5, 2024; Associated Press
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










