- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
CVS Pulling Popular Cold Meds From Shelves After Report Deems Them to Be Useless
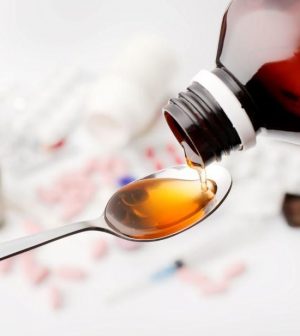
CVS Health plans to pull cold medications that contain phenylephrine from its store shelves after federal regulators determined recently that the decongestant doesn’t work.
Oral phenylephrine is an active ingredient in such well-known products as Sudafed and Dayquil. An FDA advisory committee ruled last month that the ingredient was useless in easing congestion.
“We are removing certain oral cough and cold products that contain phenylephrine as the only active ingredient from CVS Pharmacy stores,” the company told CBS MoneyWatch.
“Other oral cough and cold products will continue to be offered to meet consumer needs,” the company added.
Another drugstore chain, Walgreens, said it “follows FDA [U.S. Food and Drug Administration] regulations,” but did not say if it would stop selling the medications.
“We are closely monitoring the situation and actively partnering with the Walgreens Office of Clinical Integrity and suppliers on appropriate next steps,” a spokesperson told CBS MoneyWatch.
Medications that contain phenylephrine account for $1.8 billion in annual sales, according to FDA data.
More information
The National Library of Medicine has more on phenylephrine.
SOURCE: CBS News, Oct. 19, 2023
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










