- Comparing Whey and Plant-Based Protein: Which is Best?
- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
FDA: Wait a Month to Donate Blood After Travel to Zika-Prone Areas
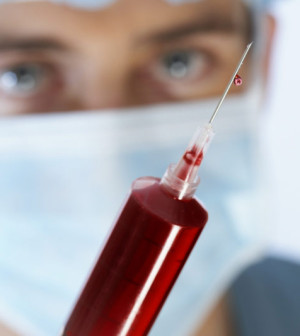
To protect the U.S. blood supply, people who’ve traveled to places where the Zika virus is prevalent, or who have symptoms that suggest infection, should wait a month before donating blood, the U.S. Food and Drug Administration announced Tuesday.
Four weeks is enough time for the virus to pass through a person’s system, the agency said.
The mosquito-borne Zika virus is thought — but not proven — to be behind an epidemic of birth defects that leave newborns with very small heads and possible brain damage.
According to the FDA, people considered to be at risk for Zika include those who have:
- Traveled to areas with active transmission of Zika virus during the past four weeks. The U.S. Centers for Disease Control and Prevention now lists 30 countries and territories in Latin America and the Caribbean as places with active Zika infection.
- Engaged in sexual contact with a person who has traveled to, or resided in, an area with active Zika virus transmission during the prior three months.
- Developed symptoms suggestive of Zika virus infection during the past four weeks.
“The FDA has critical responsibilities in outbreak situations and has been working rapidly to take important steps to respond to the emerging Zika virus outbreak,” Dr. Luciana Borio, the FDA’s acting chief scientist, said in an agency news release. “We are issuing this guidance for immediate implementation in order to better protect the U.S. blood supply.”
There have been no reports to date of Zika virus entering the U.S. blood supply, the FDA said, but the risk of blood transmission is considered likely based on the most current scientific evidence of how Zika and similar viruses are spread.
About 4 out of 5 of those infected with Zika virus do not become ill, which makes it tougher to determine whose blood might carry the pathogen, the agency noted.
The FDA announcement follows a similar move made by the American Red Cross last week, in which the organization asked potential blood donors who have traveled to Zika-affected areas to wait 28 days before giving blood.
Zika has not yet emerged in the United States, but the recommendations issued by the FDA also cover that eventuality.
The agency recommends that if an area in the country develops active Zika virus transmission, then whole blood and blood components for transfusion should be brought in from elsewhere in the United States. Blood donation centers in Zika-affected areas may continue collecting and preparing platelets and plasma if an FDA-approved, pathogen-reduction device is used.
“Based on the best available evidence, we believe the new recommendations will help reduce the risk of collecting blood and blood components from donors who may be infected with the Zika virus,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in the news release.
The FDA plans to follow up these recommendations with further guidance that will address appropriate donor deferral measures for human cells, tissues, and cellular and tissue-based products, given recent reports of sexual transmission of the virus.
The Zika virus was first identified in Uganda in 1947, and until last year was not thought to pose serious health risks. In fact, approximately 80 percent of people who become infected never experience symptoms.
But the increase in both cases and birth defects in Brazil in the past year has prompted health officials to reassess their thinking about Zika and pregnant women.
The World Health Organization now estimates there could be up to 4 million cases of Zika in the Americas in the next year.
In addition to protecting the nation’s blood supply, the FDA is also prioritizing the development of blood screening and diagnostic tests that may help identify the presence of the virus.
The agency said it is also preparing to evaluate the safety and efficacy of vaccines and medicines that might be developed to battle Zika, and reviewing technology that may help suppress populations of the mosquitoes that can spread the virus.
More information
For more information on Zika virus, and where the virus is endemic, head to the U.S. Centers for Disease Control and Prevention.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










