- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
FDA Advisors Back Moderna Shot for Older Children, Teens
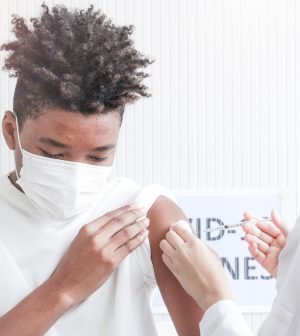
The U.S. Food and Drug Administration’s vaccine advisory panel voted unanimously on Tuesday to recommend the agency approve the emergency use of Moderna’s COVID-19 vaccine in children aged 6 to 17.
Despite the unanimous support, some panel members worried that trials of the vaccine were conducted before the emergence of the Omicron variant. They noted the vaccine would likely help prevent serious illness but not be as good at blocking mild infections, The Washington Post reported.
“We crossed a line,” when Omicron and its subvariants surfaced because now a third dose is needed for full protection, said panel member Paul Offit, a professor of pediatrics at Children’s Hospital of Philadelphia. He supported authorization — as long as a third Moderna dose is on the way.
“We’re at a different part in this pandemic,” Offit said. “I think the benefits clearly outweigh the risks, but I say that with the comfort being provided that there will be a third dose.”
Moderna told the panel it is testing a booster shot for the 6-through-17 age groups and could seek FDA authorization as early as July.
Pfizer’s COVID-19 vaccine has already been approved for this age group, so having a second option might not boost vaccinations much, at least in the 6-to-11-year-old age group. Only 29 percent of children in that age group have received the two-shot regimen of Pfizer’s shots, according to the Post.
In coming to its decision, the FDA panel analyzed Moderna’s two-dose vaccine for children aged 6 to 11 at half the strength of the adult shot, and for youth aged 12 to 17 at the adult dose. Typically, the FDA follows the recommendations of its expert panels. Once the FDA has authorized the shots, as expected, expert advisers to the U.S. Centers for Disease Control and Prevention will discuss the best use of the Moderna vaccine in older children and teens this weekend.
Moderna first asked the FDA to approve its vaccine for adolescents and older teens last June, a month after Pfizer won emergency authorization for its coronavirus vaccine to be used in 12- to 15-year-olds. The FDA then approved the emergency use of the Pfizer vaccine for kids aged 6 to 12 last October.
But regulators were worried about reports of a rare condition — known as myocarditis — that were seen mostly in young men who had been given the Moderna vaccine, so they chose to delay a decision while more research was conducted. The company said concerns about myocarditis have now subsided after further research and real-world evidence, the Post reported.
In a second meeting on Wednesday, the same FDA panel will weigh Moderna’s vaccine for children under 6 and Pfizer’s vaccine for children under 5.
There are about 18 million children under 5 in the United States and they are the only age group in the country not yet eligible for any COVID vaccines, the Post reported.
While there are concerns about whether there is enough demand for the Moderna vaccine in younger age groups, Jason Schwartz, a vaccine policy expert at the Yale School of Public Health, said any Moderna authorization could prove valuable in the long run.
“We’re still learning how these vaccines perform, both about levels and duration of protection,” Schwartz said. “We may learn over time that one vaccine is better.”
More information
Visit the U.S. Centers for Disease Control and Prevention for more on COVID vaccines for kids and teens.
SOURCE: The Washington Post
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










