- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
- Understanding the Connection Between Anxiety and Depression
- How Daily Prunes Can Influence Cholesterol and Inflammation
- When to Take B12 for Better Absorption and Energy
Pfizer Vaccine Prevents 91% of Symptomatic COVID in Young Children: FDA
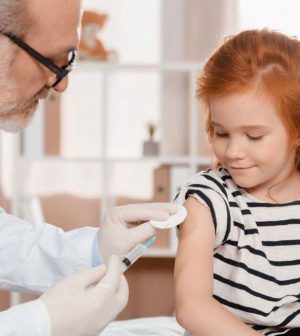
Two doses of the Pfizer-BioNTech COVID-19 vaccine is nearly 91% effective in preventing symptomatic illness in young children and brings no unexpected safety issues, according to a study posted Friday by the U.S. Food and Drug Administration.
Late Friday, the agency posted its analysis of data from Pfizer’s pediatric study and said that in nearly all models, the health benefits of administering kid-sized doses of the shot to America’s 28 million 5- to 11-year-olds far outweighs any potential side effects, the Associated Press reported.
The FDA will have a panel of independent experts offer advice on the use of the Pfizer vaccine in elementary school-age children next Tuesday, and a decision from the agency could come soon after. If the U.S. Centers for Disease Control and Prevention agrees, experts there would offer further guidance on a vaccine rollout to children under 12 — meaning that shots for young children could be administered as soon as early November, the AP said.
Children age 12 and older already have access to the Pfizer COVID-19 vaccine, but uptake by younger kids could help prevent rare cases of symptomatic illness and also greatly stem transmission, since children in this age group are thought to be conduits of new infections to adults.
The pediatric data submitted to the FDA by Pfizer was largely compiled in August and September, when the highly contagious Delta variant was already the dominant form of virus within the United States, the AP noted. Two doses of the vaccine appeared to prevent 9 out of 10 cases of symptomatic illness in young kids, with no surprises in terms of side effects, which appeared limited to sore arms, fever and achiness.
The FDA noted that the study population was not large enough to assess the risk of side effects such as myocarditis, an inflammation of the heart that has been seen in very rare cases in adults.
In four different modeling scenarios, uptake of the vaccine by young children clearly prevented many more hospitalizations than might be caused through side effects, the FDA said.
According to the AP, severe COVID is extremely rare in young children. The latest data from the American Academy of Pediatrics estimates that nearly 6.2 million American children have been infected with SARS-CoV-2 so far, resulting in a total of 630 deaths in people age 18 and under.
A large supply of millions of specially labeled pediatric doses of the Pfizer vaccine have already been stockpiled by the Biden administration, and are ready for shipment throughout the country, the AP said. More than 25,000 pediatricians and primary care providers are enlisted to administer the shots.
More information:
Find out more about COVID-19 vaccines and children at the U.S. Centers for Disease Control and Prevention.
SOURCE: Associated Press
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










