- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
FDA Warns of Counterfeit Home COVID-19 Test Kits
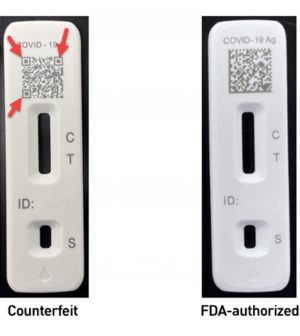
The U.S. Food and Drug Administration is warning Americans to watch out for phony at-home, over-the-counter COVID-19 tests that look a lot like the real things.
The counterfeit test kits may put you at risk of unknowingly spreading the disease or not seeking appropriate medical treatment, the agency cautions.
The phonies “are made to look like authorized tests so the users will think they are the real, FDA-authorized test,” the FDA said in a statement about the fakes. “The FDA is concerned about the risk of false results when people use these unauthorized tests.”
If you get a false reading that you don’t have the coronavirus, you could inadvertently infect others at home, at work or in medical and long-term care facilities. Also, you might not seek or could discontinue treatment for COVID-19, the agency explained.
Two fakes the FDA knows of are counterfeit Flowflex COVID-19 test kits and iHealth Antigen Rapid Test Kits — you can find more details on how to spot the fakes at the FDA statement. The package and components of the Flowflex imitation could easily mislead consumers looking for the authorized Flowflex test.
Certain red flags might help you spot the counterfeits, according to the FDA. They include:
- Poor print quality of images or text on the outside box label or in the instructions for use included in the box.
- Missing information on the outside box label for the product, such as the lot number, expiration date or barcode or QR codes.
- Grammatical or spelling errors in product labeling.
- Kit components that do not match the content description. For example, missing instructions for use, missing or unfilled components, different number of components than listed.
- The tradename for product printed on component or box labels differ from the authorized labeling found on the FDA website.
- The box label or printed instructions for use look different from the authorized labeling found on the FDA website.
The FDA has a list of authorized at-home OTC COVID-19 tests. It is not aware of any counterfeit tests distributed by federal government test distribution programs.
What should you do if you have one?
If you suspect you have a counterfeit test, do not use it. Contact the distributor or store where you bought it to tell them that you have a counterfeit test, and also inform the manufacturer of the authorized test, the agency said.
The manufacturer may ask for additional information such as photos of the packaging to further investigate the issue. After providing any requested information to the distributor and/or manufacturer, follow the manufacturer’s instructions for returning or disposing of the test.
Talk to your health care provider if you think you were tested with a counterfeit test and you have concerns about your results, the FDA advised.
If you think you had a problem with a COVID-19 test, you can report it through the FDA’s MedWatch Voluntary Reporting Form.
More information
The U.S. Centers for Disease Control and Prevention outlines what you need to know about COVID-19 testing.
SOURCE: U.S. Food and Drug Administration, news release, April 28, 2022
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










