- How Long Does Nicotine Remain in Your System?
- The Best Time of Day to Drink Bone Broth to Maximize Health Benefits
- 8 Ways to Increase Dopamine Naturally
- 7 Best Breads for Maintaining Stable Blood Sugar
- Gelatin vs. Collagen: Which is Best for Skin, Nails, and Joints?
- The Long-Term Effects of Daily Turmeric Supplements on Liver Health
- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
FDA Approves Opzelura for Atopic Dermatitis in Children
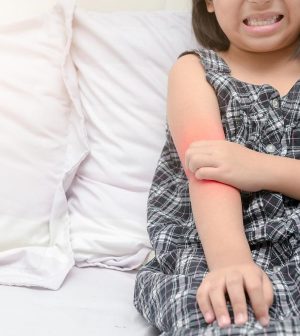
The U.S. Food and Drug Administration has approved Opzelura cream 1.5 percent (ruxolitinib) for children ages 2 to 11 years with atopic dermatitis (AD).
The approval is the first topical Janus kinase (JAK) inhibitor for the short-term, noncontinuous chronic treatment of mild-to-moderate AD in nonimmunocompromised children (age 2 years and older) whose disease is not well controlled with topical prescription therapies or when topical therapies are not recommended.
The approval is based on results from the phase 3 TRuE-AD3 trial, which evaluated the safety and efficacy of Opzelura cream in children ages 2 to <12 years with AD. The primary end point was met, with significantly more patients treated with Opzelura achieving Investigator’s Global Assessment-treatment success versus patients treated with vehicle control cream. The trial also met the secondary end point of more Opzelura-treated patients demonstrating ≥75 percent improvement in the Eczema Area and Severity Index at week 8 versus control. No new safety signals were observed, with the most common adverse reaction being upper respiratory tract infection.
“Navigating a complex condition like atopic dermatitis can be very challenging for children,” Peter Lio, M.D., from the Northwestern University Feinberg School of Medicine in Chicago, said in a statement. “With this approval, we now have a new, nonsteroidal topical option that expands how we care for kids with this chronic disease.”
Approval of Opzelura was granted to Incyte.
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










