- Could Your Grocery Store Meat Be Causing Recurring UTIs?
- Are You Making This Expensive Thermostat Error This Winter?
- Recognizing the Signs of Hypothyroidism
- 10 Strategies to Overcome Insomnia
- Could Artificial Sweeteners Be Aging the Brain Faster?
- Techniques for Soothing Your Nervous System
- Does the Water in Your House Smell Funny? Here’s Why
- Can a Daily Dose of Apple Cider Vinegar Actually Aid Weight Loss?
- 6 Health Beverages That Can Actually Spike Your Blood Sugar
- Treatment Options for Social Anxiety Disorder
Just 1 in 20 Animal Studies Yield Treatments That Make it to Humans
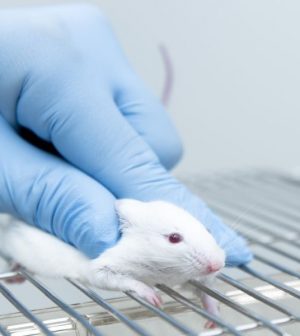
Animal studies are often considered a first step in finding new drugs and treatments for human diseases, but a new review has discovered that precious few actually produce real-world therapies.
Only 5% of therapies tested in animals wind up being approved by regulators for human use, according to an analysis of 122 articles involving 54 different diseases and 367 potential treatments.
That’s despite the fact that 86% of the time positive results in animal studies are replicated in human clinical trials, researchers said.
“Although the consistency between animal and early clinical studies was high, only a minority of therapeutic interventions achieved regulatory approval,” concluded the research team led by Dr. Benjamin Ineichen, a neurologist with the University of Zurich in Switzerland.
Research tends to follow a well-laid path — animal studies followed by early studies in humans, followed by randomized controlled clinical trials to provide solid evidence of benefit. Trial results are then submitted to regulators to have the therapy approved for humans.
About 50% of animal studies make the transition into early human studies, which are meant to show feasibility, researchers found.
But only 40% make it to randomized controlled trials, and just 5% are approved by regulators.
“Drawing from the field of clinical neurology, many therapies that have shown promise in animal studies and early trials reported as successful candidates herein, such as melatonin and mesenchymal stem cells for stroke, have not yet become standard clinical practice,” the researchers said.
“A similar pattern can be seen in other neurological diseases like Alzheimer’s disease and spinal cord injury, where there are several therapies with promising preclinical results but limited practical translation,” the team added.
The average time periods for reaching the different stages were five years from animal to human study, seven years to randomized controlled trials, and 10 years to regulatory approval, researchers found.
The new review was published June 13 in the journal PLOS Biology.
One potential explanation is that the requirements of clinical trials and regulatory approval are too strict, “causing many potentially valuable treatments to be left behind,” the researchers noted.
But they said it’s more likely that poor and inconsistent design in animal and human studies result in unreliable findings. As a result, these potential therapies don’t proceed to clinical trials.
“To improve animal-to-human translation, we advocate for enhanced study design robustness of animal and human research, which will not only benefit experimental animals but also affected patients,” the researchers said in a journal news release.
More information
The U.S. Food and Drug Administration has more about the drug development and approval process.
SOURCE: PLOS, news release, June 13, 2024
Source: HealthDay
Copyright © 2026 HealthDay. All rights reserved.










