- Double Mastectomy May Offer No Survival Benefit to Women With Breast Cancer
- Toxic Lead Found in Cinnamon Product, FDA Says
- Certain Abbott Blood Sugar Monitors May Give Incorrect Readings
- Athletes Can Expect High Ozone, Pollen Counts for Paris Olympics
- Fake Oxycontin Pills Widespread and Potentially Deadly: Report
- Shingles Vaccine Could Lower Dementia Risk
- Your Odds for Accidental Gun Death Rise Greatly in Certain States
- Kids From Poorer Families Less Likely to Survive Cancer
- Tough Workouts Won’t Trigger Cardiac Arrest in Folks With Long QT Syndrome
- At-Home Colon Cancer Test Can Save Lives
Botox Approved as Temporary Treatment for Crow’s Feet
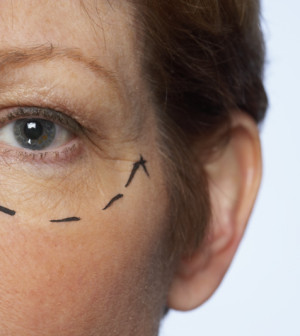

THURSDAY, Sept. 12U.S. Food and Drug Administration approval for Cosmetic Botox (onabotulinumtoxinA) has been expanded to include moderate-to-severe lateral canthal lines, the medical term for so-called “crow’s feet” lines that surround the eyes.
The drug was approved in 2002 for the temporary improvement of so-called “frown lines” between the eyebrows, the agency said in news release. The drug, made from the bacterium that causes botulism, keeps affected muscles from tightening, reducing the appearance of wrinkle lines.
The injected drug may now be given at the same time to reduce both crow’s feet and frown lines, the FDA said.
The drug’s safety and effectiveness in treating crow’s feet were established in clinical studies involving 833 adults, who were either given Cosmetic Botox or a non-medicinal placebo. Those given Cosmetic Botox showed a greater improvement in crow’s feet lines, the agency said.
The most common side effect of the drug when used for this purpose was swelling (edema) of the eyelids, the FDA said.
The non-cosmetic version of Botox has been approved to treat conditions including chronic migraine, severe underarm sweating, eyelid spasm and a certain misalignment of the eyes. The drug, in both its cosmetic and non-cosmetic forms, contains a boxed label warning of the possibility of botulism-like symptoms. These include swallowing and breathing difficulty, which could be life-threatening, the FDA said.
However, the agency said there has been no confirmed case of “toxin spread” as long as either form of Botox were used at the recommended dosages for FDA-approved reasons.
Botox is manufactured by Allergan, based in Irvine, Calif.
More information
The FDA has more about this approval.
Source: HealthDay
Copyright © 2024 HealthDay. All rights reserved.