- Most Homeless Americans Are Battling Mental Illness
- FDA Recalls Heart Failure Devices Linked to Injuries and Deaths
- COVID Does Not Spur Asthma in Kids, Study Finds
- Birth Control Pill Might Lower Odds for Sports Injuries
- Weight-loss Drug Zepbound Eases Sleep Apnea in Company Trials
- Mouse Study Shows Microplastics Migrating From Gut to Other Organs
- New Brain Target Key to Easing Tough-to-Treat Epilepsy
- Why Healthy Eating Is Key for Breast Cancer Survivors
- Placenta Plays Role in Gestational Diabetes, Study Suggests
- Some Gut Bugs May Help Lower Your Cholesterol
FDA OKs New Drug for Hard-to-Treat Colitis and Crohn’s

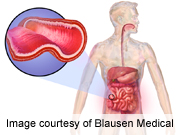
A new drug to treat adults with ulcerative colitis and Crohn’s disease has been approved by the U.S. Food and Drug Administration.
The agency said Tuesday that injections of Entyvio (vedolizumab) can be used to treat patients with moderate to severe ulcerative colitis and Crohn’s disease who have had poor responses to one or more of the current standard therapies: corticosteroids, immunomodulators, or tumor necrosis factor blocker medications.
The FDA’s approval of the new drug is based on two clinical trials of about 900 patients with ulcerative colitis, and three clinical trials of about 1,500 patients with Crohn’s disease.
Ulcerative colitis causes inflammation and ulcers in the inner lining of the large intestine and can lead to abdominal discomfort, bleeding and diarrhea. It affects about 620,000 Americans. Crohn’s can cause inflammation and irritation in any part of the digestive tract. It affects more than 500,000 Americans.
“Ulcerative colitis and Crohn’s disease are debilitating diseases that impact the quality of life of those who have these conditions,” Dr. Amy Egan, acting deputy director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research, said in an agency news release.
“Although there is no cure for these conditions, [Tuesday’s] approval provides an important new treatment option for patients who have had an inadequate response to conventional therapy to help control their symptoms,” she added.
Entyvio is a type of drug called an “integrin receptor antagonist.” These drugs improve the function of cell-to-cell interactions, the FDA said. Another type of integrin receptor antagonist — Tysabri (natalizumab) — has been linked with a rare and often fatal infection of the central nervous system called progressive multifocal leukoencephalopathy (PML).
No cases of PML occurred in patients taking part in the clinical trials of Entyvio. But the risk for patients taking the new drug remains unclear and doctors should monitor patients who are taking Entyvio for any signs of PML, the FDA said.
The FDA told Entyvio’s maker, Takeda Pharmaceuticals America Inc., that it must conduct a post-approval study to further investigate the risk of PML among patients taking Entyvio.
In the clinical trials, the most common side effects among patients taking Entyvio included headache, joint pain, nausea and fever. The most serious side effects included major infections, injection-related reactions and liver toxicity.
More information
The U.S. National Library of Medicine has more about ulcerative colitis and Crohn’s disease.
Source: HealthDay
Copyright © 2024 HealthDay. All rights reserved.