- Double Mastectomy May Offer No Survival Benefit to Women With Breast Cancer
- Toxic Lead Found in Cinnamon Product, FDA Says
- Certain Abbott Blood Sugar Monitors May Give Incorrect Readings
- Athletes Can Expect High Ozone, Pollen Counts for Paris Olympics
- Fake Oxycontin Pills Widespread and Potentially Deadly: Report
- Shingles Vaccine Could Lower Dementia Risk
- Your Odds for Accidental Gun Death Rise Greatly in Certain States
- Kids From Poorer Families Less Likely to Survive Cancer
- Tough Workouts Won’t Trigger Cardiac Arrest in Folks With Long QT Syndrome
- At-Home Colon Cancer Test Can Save Lives
Sealant Gel Approved for Eye Surgery
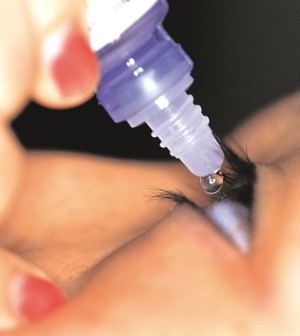

A sealant gel to prevent fluid leakage after cataract surgery has been approved by the U.S. Food and Drug Administration.
While gels such as ReSure have been approved to seal small incisions in other parts of the body, this is the first such approval for the eye, the agency said in a news release.
A cataract is a gradual clouding of the eye’s lens. By age 80, more than half of Americans have either an active cataract or have had cataract surgery, the U.S. National Institutes of Health estimates.
The ReSure Sealant kit includes two liquid solutions that are mixed together just before use. The product is designed to seal an eye incision within 20 seconds of application, the FDA said. The gel then breaks down over seven days and is naturally flushed away by the eye’s tears.
No serious adverse reactions to the gel were reported, the FDA said.
Before this approval, stitches were the only option for closing a leaking corneal incision after cataract surgery, the agency said. The product was evaluated in a clinical study of 471 adults.
The ReSure sealant is produced by Ocular Therapeutix, based in Bedford, Mass.
More information
The U.S. National Library of Medicine has more about cataracts.
Source: HealthDay
Copyright © 2024 HealthDay. All rights reserved.