- Double Mastectomy May Offer No Survival Benefit to Women With Breast Cancer
- Toxic Lead Found in Cinnamon Product, FDA Says
- Certain Abbott Blood Sugar Monitors May Give Incorrect Readings
- Athletes Can Expect High Ozone, Pollen Counts for Paris Olympics
- Fake Oxycontin Pills Widespread and Potentially Deadly: Report
- Shingles Vaccine Could Lower Dementia Risk
- Your Odds for Accidental Gun Death Rise Greatly in Certain States
- Kids From Poorer Families Less Likely to Survive Cancer
- Tough Workouts Won’t Trigger Cardiac Arrest in Folks With Long QT Syndrome
- At-Home Colon Cancer Test Can Save Lives
FDA Warns of Fires From Wart Removers

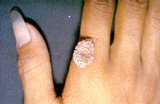
Flammable over-the-counter wart removers have started fires, injuring at least 10 people in recent years, the U.S. Food and Drug Administration says.
Since 2009, the FDA has received 14 reports about some “cryogenic” wart removers that “freeze” the growths off the skin. In several cases, combustion occurred when the products — a mixture of liquid dimethyl ether and propane — were used near a candle.
Ten people have suffered singed hair, blisters, burns or skin redness, the agency said.
“The labeling for these products clearly states that they are flammable and should be kept away from fire, flame, heat sources and cigarettes,” FDA nurse consultant Karen Nast said in an agency news release.
In three of the reports to the FDA, there was a candle nearby. But no ignition source was identified in the other 11 reports.
“This is extremely concerning, especially because people may not be aware that everyday household items like curling irons and straight irons can be hot enough to be an ignition source for these products,” Nast said.
In the incidents reported to the FDA, the wart remover dispenser generally caught fire when it was releasing the mixture, the agency said.
Nast said that even though the FDA has received only 14 reports of fires linked to cryogenic treatments, such occurrences are often under-reported. She urged consumers to tell the FDA about similar experiences. “It’s important for us to know when and how problems like this happen,” she said.
You can report device-related problems through the FDA’s MedWatch alert system.
If you use a cryogenic wart remover, use it only as directed, follow all warnings, and use it in a well-ventilated area, FDA dermatologist Dr. Markham Luke said. He noted that there are other options for treating warts.
Your doctor can remove warts using treatments such as surgical paring, laser or liquid-nitrogen freezing treatments, he said.
“The advantage is that the health care professional has been trained in providing the treatment safely and under controlled conditions,” Luke said.
Alternative over-the-counter treatments include salicylic acid, which softens or loosens warts so they fall off or are easy to remove, the FDA said.
However, Luke said warts often disappear without any treatment.
More information
The American Academy of Family Physicians has more about warts.
Source: HealthDay
Copyright © 2024 HealthDay. All rights reserved.










